The shapes around the left and right carbon atoms in the structure below are tetrahedral and linear, respectively. 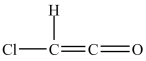
Correct Answer:
Verified
Q62: In the valence shell electron pair repulsion
Q64: The Lewis structure for the molecule below
Q65: The symbol
Q66: The structures shown below are resonance structures
Q68: The shape around each carbon atom in
Q69: A molecule that contains only one polar
Q70: The structures shown below are resonance structures
Q81: The molecular shape around the boron atom
Q90: Dibromomethane (CH2Br2)is a nonpolar molecule.
Q92: A N-O bond is more polar than
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents