An atom has the electron-dot symbol shown below. If this atom is a main group element, what charge will it form in its ionic state? 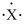
A) -1
B) +3
C) -5
D) -3
Correct Answer:
Verified
Q27: What is the chemical formula of tin
Q31: If atoms with the electron-dot symbols shown
Q34: Which chemical formula is correct?
A)Ag(CH3CO2)2
B)K2CN
C)Mg(OH)2
D)MgHCO3
E)More than one
Q36: What is the proper name for MgF2?
A)magnesium
Q37: What is the proper name for CuCl?
A)copper
Q49: What is the charge on the chromium
Q51: The correct name for Ag2O is _.
A)silver
Q55: What is the formula for the ionic
Q56: The correct name for AuI3 is _.
A)silver
Q59: Which compound has the highest melting point?
A)KCl
B)CH4
C)C6H12O6
D)H2O
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents