If the main group element has an electron-dot symbol of 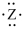 , the charge of the resulting ion will be -2.
, the charge of the resulting ion will be -2.
Correct Answer:
Verified
Q41: A cation is positively charged,and has more
Q53: Anions are formed when a neutral atom
Q62: Ionic compounds are usually solids at room
Q63: The electron configuration for the sulfide ion
Q68: The carbonate ion contains the same number
Q72: The formula for copper (II)hydroxide is Cu(OH)2.
Q78: All isotopes of an element form the
Q83: An iodide ion has 53 protons and
Q90: In crystal of an ionic compound,the cations
Q98: The ion symbol for the ferric ion
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents