Which compound could be oxidized to form the carboxylic acid below? 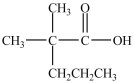
A) CH3(CH2) 5CHO
B) 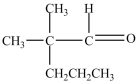
C) 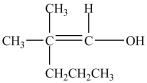
D) 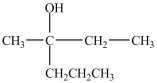
Correct Answer:
Verified
Q5: What product is formed when the compound
Q5: The Tollens reagent is which of the
Q6: What is the structure of 1-chloro-3-ethyl-2-heptanone?
A)
Q7: Which compound will give a positive result
Q8: The compound shown below belongs to what
Q9: Which compound has the greatest solubility in
Q12: Which is the structure of 3-bromo-2-methylbutanal?
A)
Q13: What is the IUPAC name of the
Q14: What is the condensed formula of the
Q15: Which compound(s)would give a positive Tollens test?
A)alcohols
B)aldehydes
C)ketones
D)carboxylic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents