Three of the four structures below represent unstable organic compounds that are not likely to exist because they violate the octet rule. Which one of the four structures represents a stable organic compound that is likely to exist?
A) 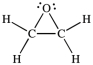
B) 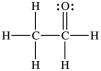
C) 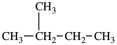
D) 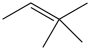
Correct Answer:
Verified
Q51: Which bonding pattern is NOT typical of
Q53: Vitamin C is a water-soluble vitamin.
Q54: Which structure properly indicates the order of
Q57: Which compounds would be expected to have
Q58: Three of the four structures below represent
Q59: The compound whose skeletal structure is shown
Q60: The two structures shown below represent the
Q62: MTBE is soluble in both gasoline and
Q69: Polar organic compounds are water soluble only
Q75: Hydrocarbons are nonpolar molecules.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents