The nuclear equation shown below represents which of the following processes? 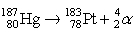
A) alpha decay of platinum
B) gamma emission by mercury
C) alpha emission by mercury
D) gamma decay of platinum
Correct Answer:
Verified
Q34: Which nuclear equation is an example of
Q35: Which nuclear equation is an example of
Q37: How many neutrons are generated in the
Q38: Which amount of radioactivity is equivalent
Q40: Which quantity represents the smallest amount
Q43: A nuclear power plant utilizes the tremendous
Q47: The initial responders to the Chernobyl nuclear
Q60: A radiation source used external to the
Q64: A positron is formed when a neutron
Q68: Hydrogen-3 is a radioactive isotope of hydrogen.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents