A 50.00-mL sample of 0.100 M Ca(NO3) 2 is mixed with 50.00 mL of 0.200 M NaF. When the system has come to equilibrium, which of the following sets of conditions will hold? The Ksp for CaF2 is 4.0 * 10-11. 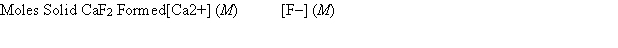
A) 10.0 * 10-3 1.3 * 10-5 M 1.3 * 10-5 M
B) 5.0 * 10-3 3.5 * 10-4 M 7.0 * 10-4 M
C) 5.0 *10-3 2.2 * 10-4 M 4.3 * 10-4 M
D) 5.0 *10-3 3.5 *10-4 M 4.3 * 10-4 M
E) 5.0 * 10-3 3.4 * 10-9 M 5.0 * 10-2 M
Correct Answer:
Verified
Q135: The Ksp for Mn(OH)2 is 2.0 *
Q136: A 200-mL solution contains 0.018 mol each
Q137: A 0.012-mol sample of Na2SO4 is added
Q138: Titrating 30.00 mL of a saturated
Q139: Calculate the solubility of Ag2SO4 [Ksp =
Q141: A 50.0-mL sample of 2.0 *10-4 M
Q142: You have 0.20 M HNO2 (Ka =
Q144: For the compound MX, Ksp is 2.00
Q150: Explain why the pH of an aqueous
Q160: Explain why we cannot directly compare Ksp
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents