The cation M2+ reacts with NH3 to form a series of complex ions as follows: 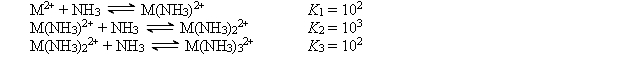 Consider an experiment in which 1.0 *10-3 mol of M(NO3)2 is added to 1.0 L of 15.0 M NH3. Calculate the equilibrium concentrations of M2+, M(NH3)22+, and M(NH3)32+
Consider an experiment in which 1.0 *10-3 mol of M(NO3)2 is added to 1.0 L of 15.0 M NH3. Calculate the equilibrium concentrations of M2+, M(NH3)22+, and M(NH3)32+
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q153: Derive the Henderson-Hasselbalch equation from the Ka
Q158: Calculate the molar concentration of uncomplexed Zn2+
Q159: A solution is formed by mixing 50.0
Q164: A solution is prepared by mixing hydrazoic
Q165: Consider a 100.0-mL sample of a 0.10
Q166: Which titration curve would result from the
Q167: Which of the following titration curves schematically
Q170: Explain how the solubility of an ionic
Q173: What is the pH of this solution?
A)
Q177: Differentiate between a formation constant and a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents