Consider the reaction 2SO2(g) + O2(g)  2SO3(g)
2SO3(g)
At constant temperature. Initially a container is filled with pure SO3(g) at a pressure of 2 atm, after which equilibrium is allowed to be reached. If y is the partial pressure of O2 at equilibrium, what is the value of Kp?
A) 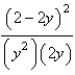
B) 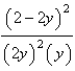
C) 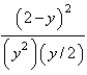
D) 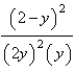
E) none of these
Correct Answer:
Verified
Q22: Nitric oxide, an important pollutant in air,
Q23: Consider the following equilibrium: N2(g) + 3H2(g)
Q24: Consider the equation 2A(g) Q25: Consider the following reaction: 2NOCl(g) Q26: Consider the following reaction: 2HF(g) ![]()


Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents