Consider the following equilibrium: 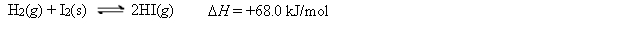 Which of the following statements about the equilibrium is false?
Which of the following statements about the equilibrium is false?
A) Removing HI as it forms forces the equilibrium to the right.
B) Adding more H2(g) increases the equilibrium constant.
C) This is a heterogeneous equilibrium.
D) If the pressure on the system is increased by changing the volume, the left side is favored.
E) If the system is heated, the right side is favored.
Correct Answer:
Verified
Q42: Explain how a given system at a
Q43: Which of the following statements is false?
A)
Q44: Raising the pressure by lowering the volume
Q48: Nitrogen gas (N2) reacts with hydrogen gas
Q49: Ammonia is prepared industrially by the
Q51: Consider the equation A(aq) + 2B(aq)
Q52: When the substances in the equation below
Q56: Which of the following statements is true?
A)
Q57: Consider the following equilibrium:
N2(g) + 3H2(g)
Q58: For a certain reaction at 25.0°C, the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents