At room temperature cyclohexane exists almost exclusively in the chair conformation (99.99%) . But at 800°C, 30% of the cyclohexane molecules exist in the twist-boat conformation. What is the value of the equilibrium constant for the following reaction at 800°C?
C6H12(chair) 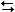 C6H12(twist-boat)
C6H12(twist-boat)
A) 0.30
B) 2.3
C) 0.23
D) 0.43
E) 0.77
Correct Answer:
Verified
Q59: Consider the equation 2A(g) Q60: Write the equilibrium constant expression for the Q61: To achieve equilibrium, the original reaction mixture Q67: Once equilibrium was established, some additional chlorine![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents