Samples of the gases H2(g) and SO2(g) have equal masses and are at the same temperature and pressure. Calculate the following:
-The ratio of the root-mean-square velocities 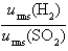 .
.
A) 180
B) 32
C) 0.18
D) 1.0
E) 5.6
Correct Answer:
Verified
Q42: A 0.234-g sample of a gas in
Q60: A 1.00-g sample of a gaseous
Q61: Four identical 1.0-L flasks contain the gases
Q62: The kinetic-molecular theory of gases does not
Q63: Samples of the gases H2(g) and SO2(g)
Q65: Consider the following gas samples: 
Q65: Calculate the temperature at which the average
Q66: Samples of the gases H2(g) and SO2(g)
Q67: Consider two 1.0-L containers, one containing He(g)
Q77: Consider separate samples of Ar(g) and Ne(g).
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents