The pressure of an ideal gas versus the number of moles at constant temperature and volume Which graph represents the plot?
A) 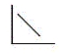
B) 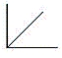
C) 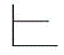
D) 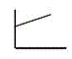
E) None of these.
Correct Answer:
Verified
Q81: Samples of the gases H2(g) and SO2(g)
Q85: Order the following according to increasing rate
Q87: The pressure of an ideal gas versus
Q87: Consider three 1-L flasks at the same
Q89: The ratio V/n versus the Kelvin temperature
Q92: Consider three 1.0-L flasks at STP. Flask
Q94: The diffusion rate of N2 gas is
Q95: Consider two gas samples: Q96: Which gas, He or H2O vapor, has Q100: Collision frequency between O2 molecules at T1![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents