The atomic mass of rhenium is 186.2. Given that 37.1% of natural rhenium is rhenium-185, what is the other stable isotope?
A) 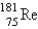
B) 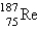
C) 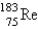
D) 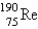
E) 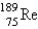
Correct Answer:
Verified
Q4: Boron naturally occurs in two isotopic forms.
Q16: For a new element, 60.78% is an
Q17: Calculate the molar mass of a sample
Q18: How many atoms of hydrogen are present
Q20: Which compound has the smallest molar mass?
A)
Q22: Find the percent oxygen (atoms) by mass
Q31: Compound X2Y is 60% X by mass.
Q32: The molar mass of the insecticide dibromoethane
Q33: Which of the following contains the greatest
Q34: A substance contains 3.024 g hydrogen, 30.97
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents