Sodium perbromate can be synthesized via the following reaction that gives off Xenon gas. NaBrO3 + XeF2 + H2O 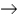 NaBrO4 + 2HF + Xe
NaBrO4 + 2HF + Xe
A reaction is started with 200.0g of NaBrO3, 200.0g of XeF2 and 100.0g of water. Assuming that the reaction goes to completion, and that all of the formed xenon gas is allowed to escape into the atmosphere, what will be the minimum mass of products and reactants remaining in the reaction vessel?
A) 326.4 g
B) 174.0 g
C) 344.9 g
D) 154.8 g
E) Not enough information given to answer the question
Correct Answer:
Verified
Q86: Consider the electrolysis of a brine
Q88: Consider the following balanced equation: A(g)
Q89: In the reaction 2A + B
Q90: Indium has two naturally occurring isotopes with
Q92: A 15-g sample of lithium is
Q92: Acetylsalicylic acid, or aspirin (C9H8O4), is an
Q93: Acetylsalicylic acid, or aspirin (C9H8O4), is an
Q94: Consider the following two unbalanced equations.
CH4
Q94: Consider a reaction in which two reactants
Q96: Triphenylene is an organic compound containing only
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents