A complex ion is a square planar complex. It has a d8 electron configuration. What is the most reasonable d orbital scheme for this complex?
A) 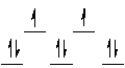
B) 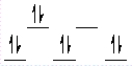
C) 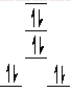
D) 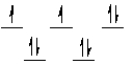
E) 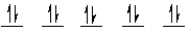
Correct Answer:
Verified
Q35: Analysis of the data from a titration
Q35: Fluoride ion ranks low in the spectrochemical
Q37: Which of the following statements about
Q39: The complex ion [TiBr4]2- is tetrahedral. How
Q39: The empirical formula of a compound with
Q41: Specify the number of unpaired electrons in
Q41: Specify the number of unpaired electrons in
Q42: How many unpaired electrons are found in
Q43: Which of the following crystal field diagrams
Q44: How many unpaired electrons are found in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents