Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species.
-Fe(OH2) 63+ (assume weak field)
A) 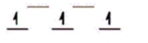
B) 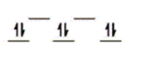
C) 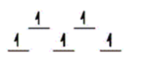
D) 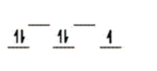
E) 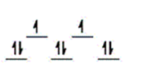
Correct Answer:
Verified
Q62: The complex ion Co(NH3)62+ (three unpaired electrons)
Q64: In _, all bonds are the same,
Q70: According to crystal field theory, how many
Q71: Oxygen is stored in mammalian tissue in
Q72: Which of the following transition metals is
Q73: The complex ion Fe(CN)64- (no unpaired electrons)
Q75: The complex ion Ni(NH3)62+ (two unpaired electrons)
Q79: Which of the following ligands are capable
Q82: Here are some crystal field representations of
Q93: Explain the toxicities of carbon monoxide (CO)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents