Aluminum metal crystallizes in a face-centered cubic structure. What is the relationship between the radius of an Al atom (r) and the length of an edge of the unit cell (E) ?
A) 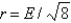
B) 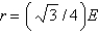
C) r = 2E
D) r = 4E
E) r = E/2
Correct Answer:
Verified
Q24: Elemental magnesium crystallizes in a face-centered cubic
Q25: Pure rubidium crystallizes in a body-centered cubic
Q25: What is the net number of face-centered
Q26: If equal, rigid spheres are arranged
Q28: Which of the following is the smallest
Q29: Chromium metal crystallizes as a body-centered cubic
Q31: A metal crystallizes in a body-centered unit
Q32: A sample of Co crystallizes in the
Q35: In cubic closest-packed solids, what percentage of
Q37: Mn crystallizes in the same cubic unit
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents