The decomposition of N2O5(g) to NO2(g) and O2(g) obeys first-order kinetics. Assume the form of the rate law is
Rate 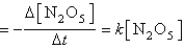 where k = 3.4 *10-5 s-1 at 25°C.
where k = 3.4 *10-5 s-1 at 25°C.
-What is the half-life for the reaction described?
A) 2.4 * 10-5 s
B) 7.4 *102 s
C) 5.9 * 105 s
D) 2.0 *104 s
E) none of these
Correct Answer:
Verified
Q33: What is the rate law for
Q34: The following initial rate data were
Q35: The decomposition of N2O5(g) to NO2(g) and
Q36: Tabulated below are initial rate data
Q37: Two isomers (A andB) of a given
Q39: The reaction
H2SeO3(aq) + 6I-(aq) + 4H+(aq)
Q40: The reaction A
Q43: The reaction
2NOBr
Q50: For the reaction aA → products, select
Q56: For the reaction aA → products, select
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents