The following question refers to the gas-phase decomposition of chloroethane: C2H5Cl products
Experiment shows that the decomposition is first order. The following data show kinetics information for this reaction. 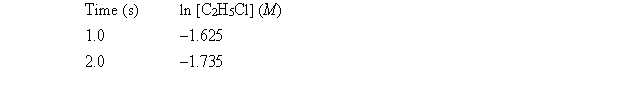 What is the rate constant for this decomposition?
What is the rate constant for this decomposition?
A) 0.22/s
B) 0.35/s
C) 0.29/s
D) 0.02/s
E) 0.11/s
Correct Answer:
Verified
Q26: For the reaction Q42: For the reaction 2N2O5(g) → 4NO2(g) + Q54: For the reaction 2N2O5(g) Q57: For the reaction aA → products, select Q58: If the reaction 2HI Q59: For the reaction aA → products, select Q61: Calculate the value of k2 where Rate Q62: Consider the reaction 3A + B Q64: Consider the reaction Q70: The following questions refer to the gas-phase![]()
3A + B +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents