Pure N2O3 was placed in a vessel and allowed to decompose:
2N2O3(g) 2N2(g) + 3O2(g)
The following data were collected in an experiment at a temperature at which 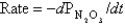 .
. 
A) Using these data, construct the appropriate plot(s), and determine the differential rate law.
B) Calculate the value of the rate constant k at this temperature.
C) Give the values of the first, second, and fifth half-lives.
D) Calculate the total pressure expected at t = 35 min.
Correct Answer:
Verified
B) 7.5 *10...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q71: The following questions refer to the gas-phase
Q78: For a reaction aA
Q79: Consider the second-order reaction aA
Q81: When ethyl chloride, CH3CH2Cl, is dissolved
Q84: The following questions refer to the reaction
Q85: The following questions refer to the reaction
Q86: The following data were collected in
Q88: The following data were collected in two
Q88: The experimental rate law for the
Q113: How many seconds would it take for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents