The hypothetical reaction
2A 2B + D
is assumed to have the mechanism 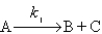
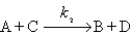 Derive the differential rate law for the production of D using the steady-state approximation.
Derive the differential rate law for the production of D using the steady-state approximation.
Correct Answer:
Verified
Q90: The following data were collected in two
Q96: What is the overall order of a
Q97: The activation energy for the reaction
Q98: For the reaction
P4(g) + 5O2(g)
Q100: The reaction 2H2O2
Q101: Which quantity is greatest?
A) acivation energy of
Q104: In the reaction
3A(g) + B(g)
Q105: Is this reaction exothermic or endothermic?
A)
Q105: For the reaction
CH3CHCH2(g) + HCl(g)
Q116: Which of the following will a catalyst
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents