A student was trying to determine the order of a chemical reaction. To accomplish this, the student graphed the concentration - time data using various plotting methodologies. The plots are shown below. 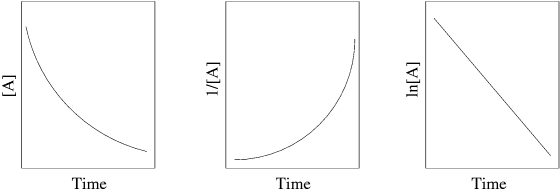 What is the order of the reaction?
What is the order of the reaction?
A) zeroth order
B) Need more data to answer the question
C) second order
D) first order
Correct Answer:
Verified
Q101: Which quantity is greatest?
A) acivation energy of
Q104: In the reaction
3A(g) + B(g)
Q105: For the reaction
CH3CHCH2(g) + HCl(g)
Q107: For the reaction
2N2O5(g)
Q109: Consider the reaction
X2Y(g)
Q110: For the reaction
2A + B
Q111: For the reaction in the presence
Q112: For the reaction
2A + B
Q112: Use the potential energy diagram shown to
Q116: Which of the following will a catalyst
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents