Using the following bond energies:  estimate the heat of combustion for 1 mol of acetylene:
estimate the heat of combustion for 1 mol of acetylene: 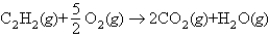
A) +365 kJ
B) 1228 kJ
C) -447 kJ
D) -1228 kJ
E) +447 kJ
Correct Answer:
Verified
Q47: What does X represent in the Lewis
Q50: Given the following information: N2 bond energy
Q50: In the Lewis structure for elemental nitrogen,
Q52: Given the following bond energies:
Q52: Which of the following molecules exhibits the
Q53: In which pair do both compounds exhibit
Q54: Use the following electronegativity values to answer
Q56: Which of the following molecules and ions
Q58: As the number of bonds between two
Q59: Using the following data reactions:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents