Estimate the bond energy of the N2 molecule. 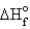 for NH3 = -46.0 kJ/mol N-H bond energy = 391 kJ/mol
for NH3 = -46.0 kJ/mol N-H bond energy = 391 kJ/mol
H-H bond energy = 432 kJ/mol
A) 1140 kJ/mol
B) 560 kJ/mol
C) 87 kJ/mol
D) 479 kJ/mol
E) 958 kJ/mol
Correct Answer:
Verified
Q29: Consider the following molecules.
I. BF3
II. CHBr3 (C
Q31: The first electron affinity value for oxygen
Q36: Which of the following molecules has a
Q37: Which of the following series is isoelectronic?
A)
Q40: Which of the following has the largest
Q42: Consider the compound crotonaldehyde, whose skeleton is
Q45: Choose the molecule with the strongest bond.
A)
Q48: In which of the following compounds does
Q49: Choose the molecule with the strongest bond.
A)
Q51: Choose the molecule with the strongest bond.
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents