Consider the compound crotonaldehyde, whose skeleton is 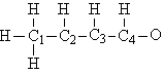 Which carbon in this molecule has tetrahedral bonding?
Which carbon in this molecule has tetrahedral bonding?
A) 2
B) 3
C) 4
D) 1
E) All have tetrahedral bonding.
Correct Answer:
Verified
Q86: Draw the Lewis structures of the molecules
Q89: Draw the Lewis structures of the molecules
Q92: Of the following, which molecule has the
Q93: Which molecule or ion violates the octet
Q94: The Cl-Kr-Cl bond angle in KrCl4 is
Q94: The molecule XCl5- has a square pyramidal
Q98: Which of the following has the Lewis
Q99: Of the following, which molecule has the
Q100: How many Lewis structures does CO32- have?
A)
Q115: Select the correct molecular structure for SF5+.
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents