A strip of copper is placed in a 1 M solution of copper nitrate, and a strip of silver is placed in a 1 M solution of silver nitrate. The two metal strips are connected to a voltmeter by wires, and a salt bridge connects the solutions. The following standard reduction potentials apply: 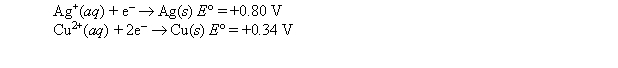 When the voltmeter is removed and the two electrodes are connected by a wire, which of the following does not take place?
When the voltmeter is removed and the two electrodes are connected by a wire, which of the following does not take place?
A) Negative ions pass through the salt bridge from the silver half-cell to the copper half-cell.
B) The silver electrode increases in mass as the cell operates.
C) Electrons flow in the external circuit from the copper electrode to the silver electrode.
D) Some positive copper ions pass through the salt bridge from the copper half-cell to the silver half-cell.
E) There is a net general movement of silver ions through the salt bridge to the copper half-cell.
Correct Answer:
Verified
Q1: How many electrons are transferred in
Q2: The following two half-reactions take place in
Q3: When the equation for the following
Q5: Which of the following is the best
Q6: The standard potential for the reaction
Q7: The reaction below occurs in basic
Q8: The following questions refer to a
Q9: Ammonium metavanadate reacts with sulfur dioxide
Q10: The following reaction occurs in basic
Q11: Determine the standard potential, E°, of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents