Refer to the galvanic cell below (the contents of each half-cell are written beneath each compartment) . 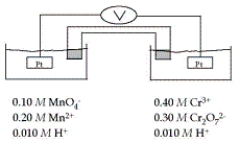 The standard reduction potentials are as follows:
The standard reduction potentials are as follows: 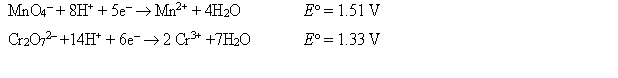
-When current is allowed to flow, which species is reduced?
A) H+
B) MnO4-
C) Cr3+
D) Mn2+
E) Cr2O72-
Correct Answer:
Verified
Q21: The reaction Cr(s) + NO3-(aq) → Cr3+(aq)
Q22: Consider an electrochemical cell with a copper
Q26: Consider the hydrogen-oxygen fuel cell where
Q29: The reaction Cr(s) + NO3-(aq) → Cr3+(aq)
Q30: Consider an electrochemical cell with a copper
Q33: Consider the following reduction potentials: 
Q34: The reduction potentials for Au3+ and
Q35: Consider the galvanic cell shown below (the
Q35: The standard free energies of formation
Q40: Refer to the galvanic cell below (the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents