Solved
Consider the Dissociation Reaction of the Acid HF S Negative?
A) the Reaction Is Expected to Be Exothermic
Multiple Choice
Consider the dissociation reaction of the acid HF. 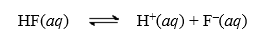 Why is S negative?
Why is S negative?
A) The reaction is expected to be exothermic, and S thus should be negative.
B) The reaction is expected to be endothermic, and thus S should be negative.
C) Each HF molecule produces two ions when it dissociates.
D) The ions are hydrated.
E) none of these
Correct Answer:
Verified
Related Questions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents