Elemental sulfur exists in two crystalline forms, rhombic and monoclinic. From the following data, calculate the temperature at which monoclinic sulfur and rhombic sulfur are in equilibrium. 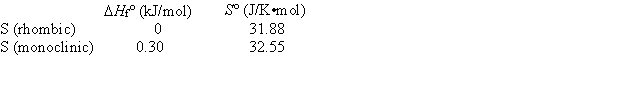
A) 0 K
B) +450 K
C) +210 K
D) -210 K
E) -450 K
Correct Answer:
Verified
Q65: When a stable diatomic molecule spontaneously
Q66: For the reaction A + B
Q67: As O2(l) is cooled at 1
Q68: The vapor pressure of Br2(l) at
Q69: In an isothermal process, the pressure
Q71: The dissociation of hydrogen H2(g) 
Q72: The vapor pressure of Br2(l) at
Q73: For the vaporization of a liquid
Q74: Consider the reaction Q75: For the process moleene(l) ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents