At 699 K, G° = -23.25 kJ for the reaction 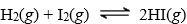 . Calculate G for this reaction if the reagents are both supplied at 10.0 atm pressure and the product is at 1.00 atm pressure.
. Calculate G for this reaction if the reagents are both supplied at 10.0 atm pressure and the product is at 1.00 atm pressure.
A) +50.0 kJ
B) +36.6 kJ
C) -36.6 kJ
D) -3.5 kJ
E) -50.0 kJ
Correct Answer:
Verified
Q78: Assuming
Q79: At constant pressure, the reaction 2NO2(g)
Q80: Consider the freezing of liquid water
Q81: Consider the reaction Q82: Given Q84: The equilibrium constant Kp for the Q85: The reaction 2H2O(g) Q86: The reaction is allowed to proceed Q87: Given Q88: The following reaction has a Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

