At room temperature cyclohexane exists almost exclusively in the chair conformation (99.99%) . But at 800°C, 30% of the cyclohexane molecules exist in the twist-boat conformation. What is the value of the equilibrium constant for the following reaction at 800°C?
C6H12(chair) 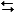 C6H12(twist-boat)
C6H12(twist-boat)
A) 0.23
B) 0.30
C) 2.3
D) 0.43
E) 0.77
Correct Answer:
Verified
Q124: The constant that connects the entropy of
Q125: For a certain process, at 328
Q126: A gas phase decomposition reaction 2AB3
Q127: For the reaction 2HF(g) 
Q128: A certain chemical reaction sets up
Q130: Given that S° = 131 J/Kmol
Q131: For a certain process, at 309
Q132: For an adiabatic process,
A) q=0
Q133: In which of the following changes is
Q134: Water gas, a commercial fuel, is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents