The oxidation of Cr3+ to CrO42- can be accomplished using Ce4+ in a buffered solution. The following data were obtained: 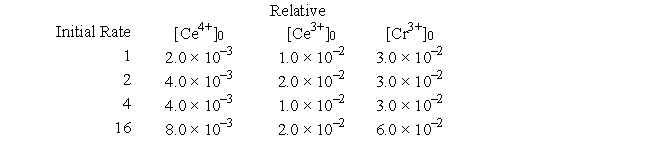
-Determine the order in the rate law of the species Cr3+.
A) -2
B) 3
C) 1
D) 2
E) -1
Correct Answer:
Verified
Q7: The following questions refer to the hypothetical
Q8: The reaction
H2SeO3(aq) + 6I-(aq) + 4H+(aq) →
Q9: The oxidation of Cr3+ to CrO42- can
Q10: A general reaction written as 2A +
Q11: For the reaction
2A + B → products
the
Q13: A general reaction written as 2A +
Q14: The rate constant k is dependent on
A)
Q15: A general reaction written as 2A +
Q16: A general reaction written as 2A +
Q17: A first-order reaction is 58% complete at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents