The decomposition of N2O5(g) to NO2(g) and O2(g) obeys first-order kinetics. Assume the form of the rate law is
Rate 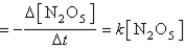 where k = 3.4 × 10-5 s-1 at 25°C.
where k = 3.4 × 10-5 s-1 at 25°C.
-What is the half-life for the reaction described?
A) 2.4 × 10-5 s
B) 7.4 × 102 s
C) 5.9 × 105 s
D) 2.0 × 104 s
E) none of these
Correct Answer:
Verified
Q25: The OH radical disproportionates according to the
Q26: Use the following initial rate data for
Q27: Two isomers (A and B) of a
Q28: For the reaction A + B →
Q29: For which of the following is the
Q31: Tabulated below are initial rate data for
Q32: For the reaction in which A and
Q33: Initial rate data have been determined at
Q34: The reaction of (CH3)3CBr with hydroxide ion
Q35: The reaction
H2SeO3(aq) + 6I-(aq) + 4H+(aq) →
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents