The reaction 2NO → N2 + O2 has the following rate law: 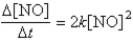 After a period of 2.0 × 103 s, the concentration of NO falls from an initial value of 2.8 × 10-3 mol/L to 2.0 × 10-4 mol/L. What is the rate constant, k?
After a period of 2.0 × 103 s, the concentration of NO falls from an initial value of 2.8 × 10-3 mol/L to 2.0 × 10-4 mol/L. What is the rate constant, k?
A) 4.0 × 10-4 M-1/s
B) 7.2 × 10-2 M-1/s
C) 1.7 × 10-4 M-1/s
D) 4.0 × 10-7 M-1/s
E) 3.6 × 10-2 M-1/s
Correct Answer:
Verified
Q42: For the reaction 2N2O5(g) → 4NO2(g) +
Q43: For the reaction 2N2O5(g) → 4NO2(g) +
Q44: For the reaction aA → products, select
Q45: For the reaction aA → products, select
Q46: If the reaction 2HI → H2 +
Q48: The reaction
2NOBr → 2NO + Br2
exhibits the
Q49: For a reaction a A → products,
Q50: For the reaction aA → products, select
Q51: The following question refers to the gas-phase
Q52: For the reaction 2N2O5(g) → 4NO2(g) +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents