The following questions refer to the reaction 2A2 + B2 → 2C. The mechanism below has been proposed: 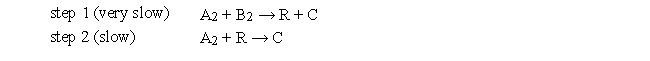
-When ethyl chloride, CH3CH2Cl, is dissolved in 1.0 M NaOH, it is converted into ethanol, CH3CH2OH, by the reaction
CH3CH2Cl + OH- → CH3CH2OH + Cl-
At 25°C the reaction is first order in CH3CH2Cl, and the rate constant is 1.0 × 10-3 s-1. If the activation parameters are A = 3.4 × 1014 s-1 and Ea = 100.0 kJ/mol, what will the rate constant be at 36°C? (R = 8.314 J/mol • K)
A) 4.9× 1014 s-1
B) 1.4× 10-3 s-1
C) 6.9× 102 s-1
D) 9.3× 10-3 s-1
E) 4.2× 10-3 s-1
Correct Answer:
Verified
Q76: The reaction A → B + C
Q77: The reaction 2NO2 → 2NO + O2
Q78: Consider the reaction
3A + B + C
Q79: At a particular temperature, the half-life of
Q80: Calculate the value of k2 where
Rate =
Q83: The reaction
2A + B → C
Has the
Q84: The following questions refer to the reaction
Q85: The following questions refer to the reaction
Q86: A reaction represented by the equation
3O2(g) →
Q113: How many seconds would it take for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents