The following questions refer to the reaction 2A2 + B2 → 2C. The mechanism below has been proposed: 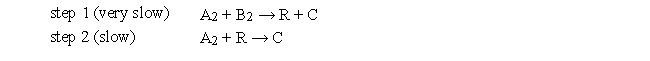
-The activation energy for the reaction H2(g) + I2(g) → 2HI(g) is changed from 184 kJ/mol to 59.0 kJ/mol at 600. K by the introduction of a Pt catalyst. Calculate the value of the ratio rate(catalyzed) /rate(uncatalyzed) .
A) 1.00
B) 0.321
C) 1.38
D) 7.62 × 1010
E) none of these
Correct Answer:
Verified
Q86: A reaction represented by the equation
3O2(g) →
Q87: The following questions refer to the reaction
Q88: The following data were collected in two
Q89: The following questions refer to the reaction
Q90: The following data were collected in two
Q92: A reaction represented by the equation
3O2(g) →
Q93: Pure N2O3 was placed in a vessel
Q94: The following questions refer to the reaction
Q95: For the reaction
P4(g) + 5O2(g) → P4O10(s)
the
Q96: What is the overall order of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents