For the reaction
CH3CHCH2(g) + HCl(g) → CH3CHClCH3(g)
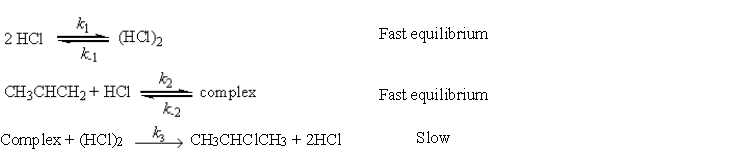
a possible mechanism is 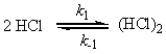
Fast equilibrium 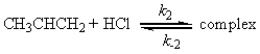
Fast equilibrium
Complex + (HCl)2 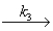
CH3CHClCH3 + 2HCl
Slow
Derive the rate law for this reaction using this mechanism.
Correct Answer:
Verified
Q105: Is this reaction exothermic or endothermic?
A)
Q106: For the reaction
2A + B → products
the
Q107: Use the potential energy diagram shown to
Q108: For the reaction
2A + B → products
the
Q109: For the reaction
2A + B → products
the
Q111: Use the potential energy diagram shown to
Q112: Use the potential energy diagram shown to
Q113: A student was trying to determine the
Q114: Consider the reaction
X2Y(g) → 2X(g) + Y(g)
At
Q115: The hypothetical reaction
2A → 2B + D
is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents