For the reaction
2NO(g) + H2(g) → N2O(g) + H2O(g)
two mechanisms, A and B, have been proposed. Derive the rate law for each proposed mechanism for the production of N2O. 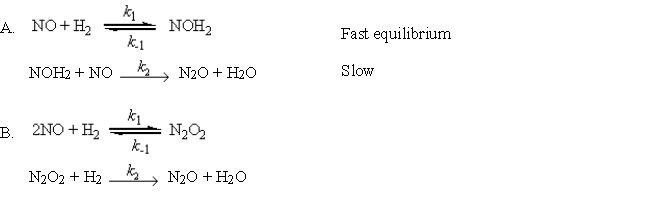
For mechanism B, use the steady-state approximation. Let rate 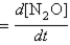
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q114: Consider the reaction
X2Y(g) → 2X(g) + Y(g)
At
Q115: The hypothetical reaction
2A → 2B + D
is
Q116: Which of the following will a catalyst
Q117: Explain what is meant by the molecularity
Q118: Rate constants are dependent upon
A) the temperature
B)
Q119: For the reaction in the presence of
Q121: Which of the following statement is/are true
Q122: Which of the following statements is/are true
Q123: _ is defined as the number of
Q124: Svante Arrhenius proposed the existence of threshold
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents