Tetracyanoethylene has the skeleton shown here: 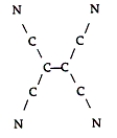 From its Lewis structure, determine the following.
From its Lewis structure, determine the following.
-How many sigma bonds and how many pi bonds are in the molecule?
A) 9 sigma and 9 pi
B) 9 sigma and 7 pi
C) 5 sigma and 8 pi
D) 6 sigma and 8 pi
E) 5 sigma and 9 pi
Correct Answer:
Verified
Q17: Consider the structure of glycine, the simplest
Q18: What is the hybridization of O in
Q19: Consider the following molecule. (Lone pairs are
Q20: Which of the following molecules contains a
Q21: As the bond order of a bond
Q23: Complete the Lewis structure for the following
Q24: Which statement about N2 is false?
A) It
Q25: Consider the following Lewis structure. (Lone pairs
Q26: The C-C-H bond angles in ethylene, C2H4,
Q27: Consider the following molecule. (Lone pairs are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents