Given the following information:
N2 bond energy = 941 kJ/mol
F2 bond energy = 154 kJ/mol 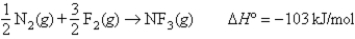
Calculate the N-F bond energy.
A) 113 kJ/mol
B) 268 kJ/mol
C) 66 kJ/mol
D) 317 kJ/mol
E) none of these
Correct Answer:
Verified
Q54: Use the following electronegativity values to answer
Q55: Consider the compound crotonaldehyde, whose skeleton is
Q56: Which of the following molecules and ions
Q57: Using the following bond energies: 
Q58: As the number of bonds between two
Q60: In which pair do both compounds exhibit
Q61: As indicated by Lewis structures, which of
Q62: How many acceptable and equivalent resonance structures
Q63: For which compound is resonance required to
Q64: Draw the Lewis structures of the molecules
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents