When molten sulfur reacts with chlorine gas, a vile-smelling orange liquid forms that is found to have the empirical formula SCl. Which of the following could be the correct Lewis structure for this compound?
A) 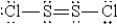
B) 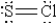
C) 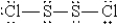
D) 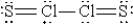
E) 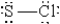
Correct Answer:
Verified
Q66: How many of the following molecules and
Q67: Select the best Lewis structure for acetone,
Q68: Which of the following is not a
Q69: How many electrons are in the Lewis
Q70: Given the following Lewis structure: 
Q72: In the Lewis structure for I3-, there
Q73: Complete the Lewis structure for the molecule
Q74: For which of the following can we
Q75: As indicated by Lewis structures, which of
Q76: The Lewis structure for H3BO3 is
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents