Which species must be polar?
A) CO 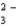
B) CH4
C) C2H4
D) CO2
E) None of the above
Correct Answer:
Verified
Q124: Refer to the SeF4 molecule.
-What is the
Q125: Which of the following is not expected
Q126: How many lone pairs of electrons are
Q127: Is the molecule polar or nonpolar?
Q128: The distance between the centers of the
Q130: Refer to the SeF4 molecule.
-What is the
Q131: A quantum chemistry program indicates that the
Q133: _, the study of the interactions of
Q134: For each of the following compounds:
A) Give
Q149: Determine whether the following oxides produce an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents