Refer to the galvanic cell below (the contents of each half-cell are written beneath each compartment) . 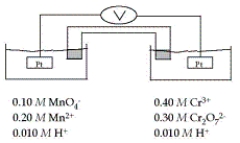 The standard reduction potentials are as follows:
The standard reduction potentials are as follows: 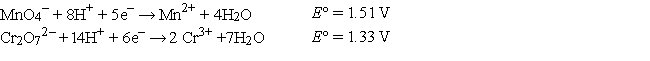
What is the oxidation state of Cr in Cr2O72-?
A) +6
B) -2
C) +12
D) -1
E) +7
Correct Answer:
Verified
Q7: What is the oxidation state of Mn
Q8: Which of the following is the best
Q9: Silver will spontaneously reduce which of the
Q10: How many electrons are transferred in the
Q11: Which metal, Al or Ni, could reduce
Q13: The following questions refer to a galvanic
Q14: Ammonium metavanadate reacts with sulfur dioxide in
Q15: Which of the following is the strongest
Q16: The following reaction occurs in basic solution:
Ag+
Q17: A strip of copper is placed in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents