Choose the correct statement(s) given the following information: 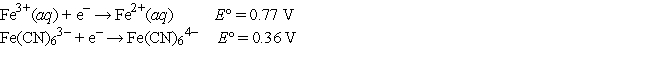
I.Fe2+(aq) is more likely to be oxidized than Fe2+ complexed to CN-.
II.Fe3+(aq) is more likely to be reduced than Fe3+ complexed to CN-.
III.Complexation of Fe ions with CN- has no effect on their tendencies to become
Oxidized or reduced.
A) I and II
B) II only
C) I only
D) III only
E) None of these is true.
Correct Answer:
Verified
Q23: In a common car battery, six identical
Q24: In which direction do electrons flow in
Q25: The reduction potentials for Au3+ and Ni2+
Q26: How many electrons are transferred in the
Q27: Refer to the galvanic cell below (the
Q29: The reaction Cr(s) + NO3-(aq) → Cr3+(aq)
Q30: Consider an electrochemical cell with a copper
Q31: Consider the galvanic cell shown below (the
Q32: The standard free energies of formation of
Q33: Consider the hydrogen-oxygen fuel cell where
H2(g) +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents