Refer to the galvanic cell below (the contents of each half-cell are written beneath each compartment) . 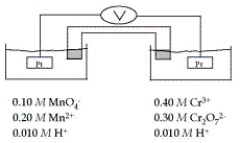 The standard reduction potentials are as follows:
The standard reduction potentials are as follows: 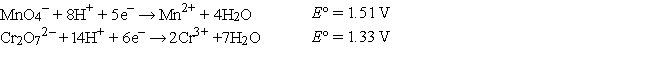
-What is the value of E°cell?
A) 2.84 V
B) 0.18 V
C) -0.18 V
D) 1.79 V
E) 2.29 V
Correct Answer:
Verified
Q32: The standard free energies of formation of
Q33: Consider the hydrogen-oxygen fuel cell where
H2(g) +
Q34: Consider the following reduction potentials: 
Q35: Consider the galvanic cell shown below (the
Q36: Refer to the galvanic cell below (the
Q38: In which of the following cases must
Q39: In which of the following cases can
Q40: Refer to the galvanic cell below (the
Q41: A concentration cell is constructed using two
Q42: Consider an electrochemical cell that has a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents