A mixture of hydrogen and chlorine remains unreacted until it is exposed to ultraviolet light from a burning magnesium strip. Then the following reaction occurs very rapidly. 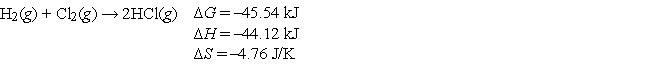 Select the statement below that best explains this behavior.
Select the statement below that best explains this behavior.
A) The reactants are thermodynamically more stable than the products.
B) The reaction is spontaneous, but the reactants are kinetically stable.
C) The reaction has a small equilibrium constant.
D) The ultraviolet light raises the temperature of the system and makes the reaction more favorable.
E) The negative value for ΔS slows down the reaction.
Correct Answer:
Verified
Q13: A gas expands isothermally and irreversibly.
-w is
A)
Q14: One mole of an ideal gas expands
Q15: Which of the following result(s) in an
Q16: One mole of an ideal gas is
Q17: In an isothermal process, the pressure on
Q19: A gas expands isothermally and irreversibly.
-ΔSsurr is
A)
Q20: One mole of an ideal gas is
Q21: 1.8 mol of an ideal gas at
Q22: Which statement is true?
A) There is always
Q23: In a certain reversible expansion, a system
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents