Consider the following hypothetical reaction at 330 K. Standard free energies of formation are given in parentheses. 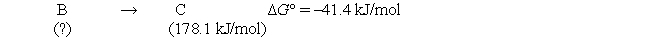 Calculate the standard free energy of formation of compound B.
Calculate the standard free energy of formation of compound B.
A) 136.7 kJ/mol
B) -314.8 kJ/mol
C) 219.5 kJ/mol
D) -219.5 kJ/mol
E) -136.7 kJ/mol
Correct Answer:
Verified
Q95: In which reaction is ΔS° expected to
Q96: Consider the gas phase reaction
NO + (1/2)O2
Q97: At 699 K, ΔG° = -23.25 kJ
Q98: Consider the gas phase reaction
NO + (1/2)O2
Q99: For the reaction
CO2(g) + 2H2O(g) → CH4(g)
Q101: Consider the gas phase reaction
NO + (1/2)O2
Q102: What will be the effect on the
Q103: Consider the following system at equilibrium at
Q104: The standard molar free energies of formation
Q105: For this system at equilibrium, how will
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents