Consider the following hypothetical reaction (at 307 K) . Standard free energies, in kJ/mol, are given in parentheses. 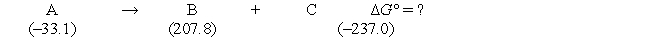 What is the value of the equilibrium constant for the reaction at 307 K?
What is the value of the equilibrium constant for the reaction at 307 K?
A) 1.5
B) 0.22
C) 1.0
D) 3.9 × 1010
E) 0.18
Correct Answer:
Verified
Q101: Consider the gas phase reaction
NO + (1/2)O2
Q102: What will be the effect on the
Q103: Consider the following system at equilibrium at
Q104: The standard molar free energies of formation
Q105: For this system at equilibrium, how will
Q107: What is the value of ΔH for
Q108: The equilibrium constant Kp (in atm) for
Q109: Consider the reaction
2SO2(g) + O2(g) 
Q110: Consider the following system at equilibrium at
Q111: Given the following free energies of formation:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents