At room temperature cyclohexane exists almost exclusively in the chair conformation (99.99%) . But at 800°C, 30% of the cyclohexane molecules exist in the twist-boat conformation.What is the value of the equilibrium constant for the following reaction at 800°C?
C6H12(chair) 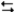 C6H12(twist-boat)
C6H12(twist-boat)
A) 0.23
B) 0.30
C) 2.3
D) 0.43
E) 0.77
Correct Answer:
Verified
Q122: For the reaction 2HF(g) Q123: Consider the reaction below at 25°C, for Q124: For an adiabatic process, Q125: The constant that connects the entropy of Q126: Which of the following statements is/are true Q128: Calculate w and ΔE when 1 mol Q129: A 1.00-mol sample of an ideal monatomic Q130: For the reaction CO(g) + 2H2(g) Q131: Given that S° = 131 J/Kmol for Q132: In the equation P1V1![]()
A) q = 0
B)

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents